The Electron Theory
Sub-elements of an Atom
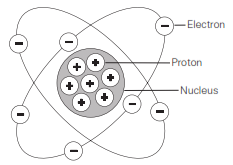
All matter is composed of molecules which are made up of a combination of atoms. Atoms have a nucleus with electrons orbiting around it. The nucleus is composed of protons and neutrons (not shown). Most atoms have an equal number of electrons and protons. Electrons have a negative charge (-). Protons have a positive charge (+). Neutrons are neutral. The negative charge of the electrons is balanced by the positive charge of the protons. Electrons are bound in their orbit by the attraction of the protons. These are referred to as bound electrons.
Free Electrons
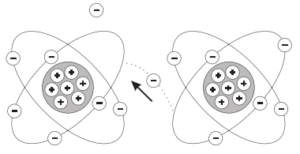
Electrons in the outer band can become free of their orbit by the application of some external force such as movement through a magnetic field, friction, or chemical action. These are referred to as free electrons. A free electron leaves a void which can be filled by an electron forced out of orbit from another atom. As free electrons move from one atom to the next an electron flow is produced. This is the basis of electricity.
Two Types of Electricity
Static Electricity
Static electricity is when electrical charges build up on the surface of a material. It is usually caused by rubbing materials together. The result of a build-up of static electricity is that objects may be attracted to each other or may even cause a spark to jump from one to the other. For Example rub a balloon on a wool and hold it up to the wall.
Before rubbing, like all materials, the balloons and the wool sweater have a neutral charge. This is because they each have an equal number of positively charged subatomic particles (protons) and negatively charged subatomic particles (electrons). When you rub the balloon with the wool sweater, electrons are transferred from the wool to the rubber because of differences in the attraction of the two materials for electrons. The balloon becomes negatively charged because it gains electrons from the wool, and the wool becomes positively charged because it loses electrons.
Current Electricity
Current is the rate of flow of electrons. It is produced by moving electrons and it is measured in amperes. Unlike static electricity, current electricity must flow through a conductor, usually copper wire. Current with electricity is just like current when you think of a river. The river flows from one spot to another, and the speed it moves is the speed of the current.
With electricity, current is a measure of the amount of energy transferred over a period of time. That energy is called a flow of electrons. One of the results of current is the heating of the conductor. When an electric stove heats up, it’s because of the flow of current.
There are different sources of current electricity including the chemical reactions taking place in a battery. The most common source is the generator. A simple generator produces electricity when a coil of copper turns inside a magnetic field. In a power plant, electromagnets spinning inside many coils of copper wire generate vast quantities of current electricity.
Types of Current Electricity
Direct Current (DC)
Alternating Current (AC)
There are two main kinds of electric current, which is easy to remember.
Direct Current (DC) – Direct current is usually the energy you get from a battery. Alternating Current (AC) is like the plugs in the wall.
The differences between the two are the following:
DC is a flow of energy and can be stored in batteries. The AC can turn on and off, and AC reverses the direction of the electrons. AC cannot be stored and when the source stops running it will stop flowing.
