Electric Charges
Neutral State of an Atom
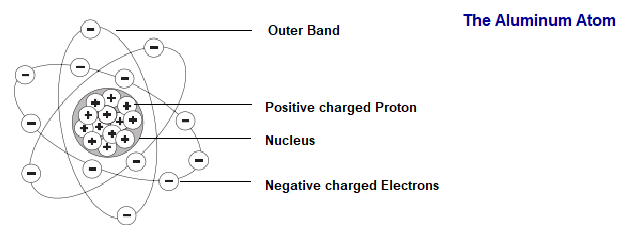
Elements are often identified by the number of electrons in orbit around the nucleus of the atoms making up the element and by the number of protons in the nucleus. A hydrogen atom, for example, has only one electron and one proton. An aluminum atom (illustrated) has 13 electrons and 13 protons. An atom with an equal number of electrons and protons is said to be electrically neutral.
Positive and Negative Charges
Electrons in the outer band of an atom are easily displaced by the application of some external force. Electrons which are forced out of their orbits can result in a lack of electrons where they leave and an excess of electrons where they come to rest. The lack of electrons is called a positive charge because there are more protons than electrons. The excess of electrons has a negative charge. A positive or negative charge is caused by an absence or excess of electrons. The number of protons remains constant.

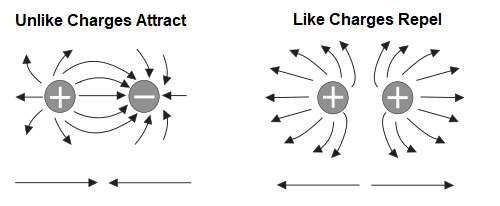
Attraction and Repulsion of Electric Charges
Electrons in the outer band of an atom are easily displaced by the application of some external force. Electrons which are forced out of their orbits can result in a lack of electrons where they leave and an excess of electrons where they come to rest. The lack of electrons is called a positive charge because there are more protons than electrons. The excess of electrons has a negative charge. A positive or negative charge is caused by an absence or excess of electrons. The number of protons remains constant.